Clausius-Clapeyron Equation
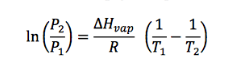
The two-point form of the Clausius-Clapeyron equation relates the vapor pressures of a substance at two different temperatures, which depends on the enthalpy of vaporization of the substance. The equation can also be used to calculate the enthalpy of vaporization if the vapor pressures at two temperatures are known.
Enter any four variables out of temperature 1, temperature 2, pressure 1, pressure 2, and enthalpy of vaporization to calculate the missing fifth variable: